Photo: nature.com
"Promising Phase 1/2 Trial of VT3989 Targets Mesothelioma in Solid Tumors"
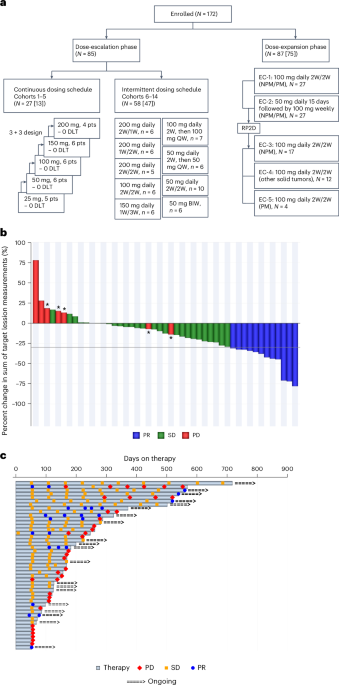
A promising Phase 1/2 trial of VT3989 targets mesothelioma, a rare and aggressive cancer, addressing a significant unmet medical need with potential advancements in drug development.
- The Phase 1/2 trial of VT3989 focuses on treating mesothelioma, a rare cancer linked to high rates of Hippo pathway alterations, indicating a critical need for innovative therapies.
- Participants in the trial, conducted at the MD Anderson Cancer Center, will receive VT3989, an enzyme inhibitor aimed at disrupting the YAP1 protein pathway involved in cancer progression.
- Current treatment options for mesothelioma primarily include chemotherapy, surgical resection, and immune therapies, underscoring the urgency for effective new treatments as emphasized by the European Society for Medical Oncology.
Why It Matters
This trial represents a pivotal advancement in cancer treatment, particularly for mesothelioma patients facing limited options. Success in drug development could redefine therapeutic strategies and improve outcomes for this challenging disease.